Planck's quantum theory-Jayam chemistry learners
Postulates of Planck's quantum theory:
Introduction to Planck's quantum theory:
Planck’s
quantum theory explains the particle nature of light with quantization.
James Clerk
Maxwell interpreted light as propagating wave of electric and magnetic field
couple.
The
mathematical equations of Maxwell and the electromagnetic study of Hertz
successfully proved the wave character of light with a phenomenon such as
diffraction and interference.
 |
| Explanation of Planck quantum theory |
The classical physics depictions of light could not explain phenomena such as black body radiations and the photoelectric effect.
The limitations of classical physics in explaining the ultraviolet catastrophe of black bodies laid the foundation for Planck’s experiments on the energies of oscillating particles.
Planck’s
quantum theory proposed the quantum nature of electromagnetic radiant energies.
It
explained the particle character of light that deals with the photoelectric
effect and black body radiations.
It is the
basic theory of quantum mechanics. And it paved the way for the dual character
of matter.
Previously,
scientists considered that matter and energy were two unrelated separate
entities with unique behavior.
Later, they accepted the dual behavior of matter and light proposed by de-Broglie.
Table of contents:
Introduction to Planck's quantum theory
What are the postulates of Planck's quantum theory?
Video explanation of Planck's quantum theory?
Different forms of Planck quantum theory?
Mind map of Planck's quantum theory
Overview of Planck's quantum theory
Evidence in support of Planck's quantum theory
Ultraviolet catastrophe-solved by Planck's quantum theory
Applications of Planck's quantum theory
Limitations of Planck's quantum theory
Numerical problems of Planck's quantum theory
Frequently asked questions and answers on Planck's quantum theory
Our e-book
Grab a colorful e-book of Planck quantum theory e-book available at our online store, "Jayam chemistry adda."
What are the postulates of Planck’s quantum theory?
A body can absorb and emit discontinuously tiny packets of energy.
Each small energy packet is known as a quantum. Uniquely the term photon denotes light energy.
Energy associated with quantum varies directly with the frequency of electromagnetic radiation.
E = nhν
E= Energy
of quantum
h= Planck
constant
ν=
frequency of electromagnetic radiation
n= non-zero
positive integer
Alternatively,
quantum energy is the integral multiple of the product of Planck's constant and
frequency of light radiation.
Hence, a body can accept or release whole number multiples of quantum. For example- an object can give or take 1hν, 2hν, or 3hν, nhν units of energy.
Energy in fractions of a quantum can neither emitted nor absorbed. For example- an object cannot transmit 1/2hν, 3/2hν, or 5/4hν units of energy.
Video explanation of Planck's quantum theory postulates
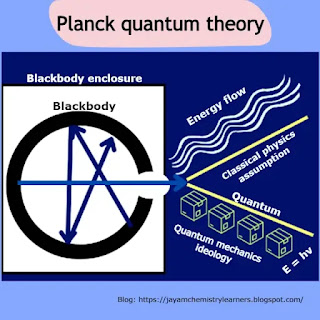
Planck quantum theory
defines the magnitude of photon energy. Here is an image with three figures.
Figure 1 shows energy interaction as a particle when stuck with a matter. Quantum is the name of an energy cell that exchanges between the bodies.
In figure 2, the
oscillatory charged ions intake energy packets which enhance their kinetic
energy. Afterward, the body releases excess energy bundles to the surroundings
to attain a stable state. The photon transfer between the body and the
surrounding occurs intermittently in regular periodic intervals.
Figure 3 shows all the
different forms of the Planck quantum formula, which we discussed in the blog
post.
In this way, Planck's quantum theory brought a revolutionary change in the classical supposition of radiant energies.
Please click the image to view the video which explains the Planck quantum theory.
Additional reference:
Multiple choice questions with answers on Planck's quantum theory
Different forms of Planck’s quantum equation:
Planck’s quantum- frequency equation:
To measure
the frequency of a photon, we can use this formula.
E ∝ ν
E = hν
It shows
the energy of a photon varies directly with its frequency.
So,
low-frequency radiations contain low energy. Conversely, high energetic
radiations possess high frequencies.
It
indicates the frequency of radiation expresses its associated energy.
Planck’s quantum- wavelength equation:
Evidently,
we have
ν ∝ 1/λ
ν =c/λ
Where,
c= velocity of
light in vacuum
λ= wavelength of the electromagnetic
radiation
The photon
energy in terms of wavelength of a light radiation is
It
indicates that the energy of a photon varies inversely with the wavelength of
the light radiation.
Radiations
with longer wavelengths have lower energies. And shorter wavelength radiations
are highly energetic.
With this
equation, we can calculate the energy of light radiation from its wavelength
data.
 |
| Planck quantum law mathematical explanation |
Planck’s quantum- wavenumber equation:
We know
that,
Wavenumber =
1/wavelength
ῡ = 1/λ
The energy of a single photon in terms of its wavenumber is E = hcῡ
E/hc = ῡ
Where,
ῡ =
wavenumber of the light
It
indicates the wavenumber of the light radiations varies directly with its
photon energy.
A photon
with a higher wavenumber has higher energy. Conversely, lower energy photon has
lower wavenumber.
To
conclude, Planck's quantum theory helps calculate the photon energies from the
known data of wavelength, frequency, or wavenumber of electromagnetic
radiation.
Mind map of Planck quantum theory
 |
| Mind map of Planck quantum theory |
Planck's quantum
theory described radiant energy as an energy particle called quantum to solve
the ultraviolet catastrophe. It came up with empirical relation to enumerate
the energy distribution of blackbody emissions.
The energy particle
size is the multiplicative product of Planck's constant and blackbody radiation
frequency.
The universal fixed
numerical quantity played a crucial role measure the quantum energy precisely.
This mind map
discusses the basic principle of Planck quantum theory along with its
applications and limitations.
Overview of Planck's quantum theory:
Planck's
quantum theory explains the quantum mechanical phenomenon of thermal
electromagnetic radiations emitted by black bodies.
It
determines the spectral density of black body radiations at a constant
temperature when there is no net flow of matter or energy between the black
body and its surrounding.
It elucidates
the interaction of electrically charged sub-atomic particles with
electromagnetic radiations.
Max Planck
proposed an imaginary electrically charged oscillating object in the cavity of
a rigid body in thermal equilibrium condition.
He
determined that the oscillating object emits only tiny energy portions at
varying temperature conditions.
He
discovered the term "quantum" to denote the minimum amount of energy
emitted or absorbed by the oscillator.
The amount
of energy less than a quantum is neither emitted nor absorbed.
The black
body emits radiations from a small hole in the opaque walls of an enclosure at
uniform temperature conditions.
At thermal
equilibrium, the radiation released from the cavity of the black body
experimentally agreed with the quantum assumptions of Max Planck.
So, the
incremental energy changes of the black body emissions showed their spectral
intensity from low frequency to higher frequency radiations.
It corrects the substantial variation of
radiant energies as mentioned by classical physics.
Additional reference:
A brief synopsis of blackbody and its radiation
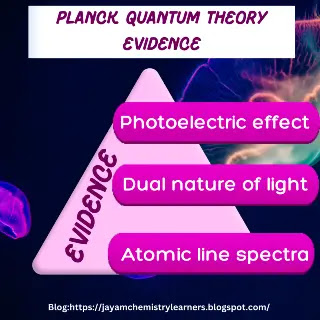
Evidence in support of Planck's quantum theory:
It
successfully explained the radiation emissions from the black bodies. And it
solved the limitation of the Rayleigh-Jeans law.
When light
radiation interacts as a wave with the metal surface, it cannot eject the
photoelectron. Hence, the photoelectric effect proves the particle nature of
the light.
It formed
the basis of the dual nature of light explained by de-Broglie in 1923.
Light shows
particle character when interacting with matter. And it exhibits wave character
during its propagation.
Also,
Bohr's atomic model relies on the energy quantization principle to explain the
stability of the atom.
And it
explained the line spectra of hydrogen atoms based on the discontinuous photon
emissions. It confirmed the existence of discrete packets of energy in light
radiation.
Based on all these applications of Planck's quantum theory, Max Planck is known as the father of quantum theory.
Additional reference:
What is the difference between a photon and a quantum?
Ultraviolet catastrophe- solved by Planck’s quantum theory:
Gustav Kirchhoff
envisioned a hypothetical object capable of absorbing all wavelength radiations
that fall on it. And he named it black body.
The black
body re-emits all absorbed radiations upon heating. These are black body
radiations.
 |
| Ultraviolet catastrophe |
Experiments of Rayleigh and Jeans on black bodies ended with the assumption of ultraviolet catastrophe.
Rayleigh-Jeans
law showed the variation of light intensity with wavelength at a particular
temperature (T).
It suggests the
radiated energy per unit frequency varies directly with ν2.
Hence, the
radiant energy sum gives an infinite value at higher frequencies. It exhibits a
nonphysical spectrum of boundless black body radiant energies that grows
continuously in the ultraviolet region. It is known as the ultraviolet
catastrophe.
In this way, the theoretical assumption of Rayleigh-Jeans law deviated from practical observations of black body emissions at shorter wavelengths.
To explicit this divergence, Max Planck considered the discrete energy emissions by the black bodies instead of continuous energy discharges.
Hence, Max
Planck explained the cause of ultraviolet catastrophe by quantizing the energy
of black body radiations.
He
expressed the energy of electromagnetic radiation in terms of its frequency at
different temperature conditions.
At low
temperatures, the black body emits less energetic radiations.
.webp) |
| Blackbody curve |
With the
increase in temperature, the black body expels high energetic radiations.
It indicates the frequency of black body
radiations varies directly with the temperature.
As
mentioned earlier, the black body cannot lose energy continuously at each temperature.
Instead, it
expels discrete amounts of energy at periodic intervals corresponding to the
temperature change.
The
assumption of erratic energy emissions by the black body solved the heavy
energy change in the ultraviolet region as predicted by Rayleigh-Jeans law
leading to ultraviolet catastrophe.
It solved
the puzzle of black body radiations not explained by classical mechanics.
Rather than
a smooth curve, Planck's assumptions gave an inverted U-shaped curve for the intensity
of black body radiations. And it matches closely with the experimental results.
The
spectral energy of objects rises with temperature initially. It continues to a
particular temperature where the intensity of emitted light peaks at maximum.
After
attaining the peak value, the energy of the black body drops slowly with an
increase in temperature. It is due to the release of more low-energy packets.
It reduces
the chances of energy accumulations in the ultraviolet region.
Applications of Planck’s quantum theory:
Planck's
quantum theory is the fundamental theory of quantum mechanics.
The
semiconductor-based electronic gadgets follow the quantum nature of matter.
Fiber optic
telecommunications and laser devices involve the phenomenon of photon
interaction with matter.
Atomic
clocks fitted in satellites for GPS navigation follow quantum physics.
MRI
(Magnetic resonance imaging) scan works on the quantum nature of light and
matter.
Limitations of Planck’s quantum theory:
It
described the energy emissions of periodic systems. It does not apply to
non-periodic objects.
It is
silent about the relative intensities of spectral lines.
It proposed
the electron as a spin-less oscillating object. And it did not explain the spin
motion of the electron.
Numerical problems on Planck quantum theory:
Question-1: Calculate the energy of a photon at 525 nm.
Solution:
The wavelength of
photon = 525 nm
The formula to measure
the energy of photon is
E = 19.878 x 10-26/λ
E = 19.878 x 10-26/525
x 10-9 joules
E = 3.78 x 10-19
joules
Question-2: Find the number of photons of light at 4000 pm having one joule of energy
Solution:
Energy of photon = 1 J
Wavelength of light
radiation = 4000 pm = 4000 x 10-12 m
The formula to
calculate the number of photons is E = nhc/λ
n = λE/hc
n = (4000 x 10-12
m) x 1J/19.878 x 10-26 jm
n = 2.0122 x 1016
Question-3: A 242 nm electromagnetic light can ionize a sodium atom. What is the ionization energy of sodium in KJ/mole?
Solution:
The wavelength of
light radiation = 242 nm = 242 x 10-9 m
The formula to measure
the ionization energy for one sodium atom is
E = 19.878 x 10-26
/ λ
E = 19.878 x 10-26
/ 242 x 10-9 joules
E = 0.0821 x 10-17 joules
Ionization energy of
one mole of sodium atoms = Ionization energy of one sodium atom x Avogadro’s
number
Ionization energy of
one mole of sodium atoms = (0.0821 x 10-17) x (6.023 x 1023)
Ionization energy of
one mole of sodium atoms =494 KJ/mole
Question-4: A nitrogen laser produces electromagnetic radiation at 337.1 nm and emits 5.6 x 1024 photons. Calculate the energy of the radiation.
Solution:
Wavelength of
electromagnetic radiation = 337.1 nm
Number of photons
emitted from nitrogen laser = 5.6 x 1024
The formula to measure
the radiation energy is
E = nhc/λ
E = (5.6 x 1024)
x (19.878 x 10-26) / 337.1 x 10-9
E = 3.302 x 106 Joules
Question-5: Calculate the photon energy having a frequency of 4.5 x 1012 Hz
Solution:
The frequency of photon = 4.5 x 1012 Hz
The formula to measure the photon energy is E = hν
E = (6.626 x 10-34) x (4.5 x 1012)
E = 2.98 x 10-21 joules
Question-6: What is the wavelength of light radiation if it has 8 x 1015 Hz frequency?
Frequency of light radiation = 8 x 1015 Hz
The formula to measure the wavelength of photon is
λ = c/ν
λ = 3x 108
/ 8 x 1015
λ = 37.5 nm
Our e-book:
If you want to own a copy of the e-book on Planck quantum theory numerical problems, click on the link.
Frequently asked questions and answers on Planck's quantum theory concept:
Question-1: Who discovered Planck's quantum theory?
In 1900,
Max Planck explained the particle nature of light with his theory known as
Planck's quantum theory.
It solved
the mysteries of black body emissions in the ultraviolet region.
Planck
proposed that light consists of discrete packets of energy called quantum.
Planck’s
quantum theory explains the interaction of light as a particle with matter.
Question-2: What is Planck's quantum formula?
Planck's
quantum formula shows the relationship of a photon with the frequency of light.
E ∝ ν
E = hν
According
to it, the energy of light radiation varies directly with its frequency.
So, an
increase in the frequency of emitted or absorbed light radiation increases its
energy. And vice versa.
Question-3: What do you mean by a quantum?
Excluding light, quantum is an energy bundle
for all kinds of energy. The packet of light energy is called a photon.
According to Planck's quantum theory, the
quantum is the minimum amount of energy emitted or absorbed by the body.
Energy less than a quantum is neither absorbed
nor emitted.
Max Planck determined that an oscillating
object can accept or release discrete packets of energy.
The energy transfer is not continuous, instead
follows a periodic change according to the surrounding energy.
Question-4: How does Planck's quantum theory explain the photoelectric effect?
The photoelectric effect is the process of
electron emission from the metal surface when light radiation of minimum
frequency incident on it. The required minimum frequency is known as the
threshold frequency.
According to Planck's quantum theory, the light
energy and frequency varies directly with each other.
Hence, the context of minimum frequency in the
photoelectric effect denotes the energy of electromagnetic radiation.
Besides, Planck proposed that an object absorbs
or emits definite minimum energy called quantum.
All these together hint at the discrete packets
of energy a body transfers with its surrounding. This threshold energy is the
principal factor to initiate the photoelectric effect.
Question-5: Why did Planck introduce quantum theory?
Classical physics assumed that the black body
emits energy continuously. It leads to the excess release of radiant energy in
the ultraviolet region at shorter wavelengths.
It is named ultraviolet catastrophe. This
theoretical assumption diverges from the practical observations of black body
radiations.
Max Planck put forward his quantum theory to
explain the drawbacks of black body emission proposals of classical physics.
Planck elucidated that the black body emits
energy discontinuously in small installments known as quantum rather than
continuously.
This concept of discrete photon emissions by
black body radiations matched closely with the experimental results and
corrected the limitations of classical physics.
Check your knowledge:
Fill in the blanks:
Q.1. The
quantized energy packet of oscillatory electron is called______________
Q.2. Photon
energy varies ____________________with its radiation frequency
Q.3. Excess
energy release in the ultraviolet part of the electromagnetic spectrum is
called __________________
Q.4. Light
is a propagating wave of light and magnetic fields couple. It was proposed
by__________
Q.5.
Discontinuous energy emissions of LED bulb are called as________________
Short questions and answers:
1. What is the
difference between a quantum and a photon?
2. What is the
Planck wavelength equation?
3. Write a
single application and limitation of Planck quantum theory.
Fill in the
blanks answers:
Q.1.
Answer: Quantum
Q.2.
Answer: Directly
Q.3.
Answer: Ultraviolet catastrophe
Q.4.
Answer: James Clerk Maxwell
Q.5.
Answer: Photon
Conclusion:
We hope you got all the information about the topic postulates of Planck quantum theory in our blog post. And kindly check our additional resources links highlighted in the blog post for more details.
And do check our chemistry-dedicated social media channels for more image content on the Planck quantum theory.
And you can get in touch with us on the social channels provided.