Fine structure of the hydrogen atom-chemistry learners
Hydrogen fine structure
Introduction to hydrogen fine structure
In our busy
mechanical life, maybe you spare some time to enjoy the sunrise or sunset to
take the joy of inexplainable moments of natural patterns that feast your eyes.
You experience the outreach of sunrays on earth that tune your mind to amicable
mood modes. There are a lot of scientific mysteries hidden behind these nature
scenarios that we do not know. Yeah, it is true. The sun rays reaching the earth
carry spectra of many chemical elements including hydrogen. Today, in this blog
article, we will discuss the fine structures of the hydrogen atom.
The
standard model of physics explains the basic building blocks of the universe.
According to this model, there are four forces that govern the universe. They
are electromagnetism, strong force, weak force, and gravitational force. Among
them, electromagnetism involves the interaction of the electric and magnetic
fields and the light radiations carry it.
Our topic
of discussion, the hydrogen fine structure, results from the influence of the
intrinsic electromagnetic force of the atom with the photons. It involves the
interaction of quantum mechanical spin with the electron's orbital motion.
 |
| Fine structure of the hydrogen atom |
What is the fine structure of a hydrogen atom?
The
splitting of the main spectral line into two or more components with a slight
variation in wavelength in the magnetic field is called fine structure in
spectroscopy. It means that, in the magnetic field, the electron energy splits
to give its sub-states. The electron transitions from these substituent energy
levels give additional spectral lines. These are known as fine structures of
the main spectral line. The hydrogen spectrum exhibiting the fine structured
lines is known as the hydrogen fine spectrum.
Hydrogen is
the lightest element with a single electron in the periodic table. The hydrogen
atom absorbing external energy shows the excitation of its electron. In a
hydrogen atom, the transition of electrons between the two discrete stationary
energy levels results in the emission of photons of definite wavelengths. It
shows spectral lines in the hydrogen spectrum. Unlike ordinary spectrometers, a
high-resolution spectrometer epitomizes the main spectral line splitting into its
constituents with a slight variation in their wavelengths. The splitting of
spectral emission lines of the hydrogen are known as the hydrogen fine
structures.
 |
| The scientific explanation image for hydrogen fine structure. |
The
cleavage of the main spectral line of the hydrogen atom is due to the influence
of spin-orbit coupling. The interaction of spin electron magnetic moment with
the magnetic field of electron’s relative motion gives the hydrogen fine
structures.
Additional reference:
A PowerPoint presentation on the hydrogen fine structure
An infographic explaining the types of the hydrogen spectrum
The hydrogen emission spectrum- PowerPoint presentation
History of the hydrogen fine structure
An atom is
the smallest indivisible unit of matter, which forms all the chemical elements
that exist in the universe. The protons and neutrons are the fundamental
particles of the nucleus, and they bind together to form the central massive
positive part of the atom. The electrons are the lightest charged leptons bound
to the nucleus with the electrostatic force of attraction to make the atoms.
Since the
1550s, the smelting of ores imparted characteristic colors to the flame. It
served as an identification method to find chemical substances in the ores.
Since then, spectral studies revealed that the atomic spectrum is a unique
characteristic identity for each atom as fingerprints in humans.
The study
of the hydrogen spectrum has a remarkable place in astronomy due to its
abundance in the universe. Many scientists in the 19th century observed the
hydrogen spectral lines while studying the solar spectrum. Similarly, in 1887,
Michelson and Morely depicted precisely the absorption trends and spectral
emission lines of hydrogen. They demonstrated the small shifts in hydrogen energy
levels and the additional spectral emission lines in the hydrogen spectrum.
More clearly speaking, they explained the fine structure of the hydrogen atom.
During the
19th century, several atomic theories tried to explain the structure of the atom. But the Bohr model of the atom successfully interpreted the arrangement
of electrons around the nucleus.
Additional reference:
What is the history of the hydrogen spectrum?
What is the hydrogen emission spectrum?
Bohr atomic model overview:
Bohr's
atomic theory explains the planetary motion of electrons around the nucleus in
permitted stationary orbits known as the energy levels. Only those energy
levels are allowed that satisfy Bohr's angular momentum condition. And the
electron transition between these granted stationary energy levels gives the
absorption and the emission spectral lines in the atom.
It gave a
groundbreaking theoretical explanation for the spectral emissions of hydrogen
by introducing quantized energy shells. It also proved the existence of
discrete energy levels with the line spectra of the hydrogen atom. Bohr’s
predictions on energy levels of the hydrogen atom closely matched those
mentioned by Michelson and Morely. There are a few differences between these
two theories. According to Bohr atomic model, a single spectral line ensues in
a single electron transition. But according to Michelson and Morely, a single
electron transition can give more than one spectral line. Bohr's theory did not
explain the exact reason for the spectral line splitting.
In 1913,
Neil Bohr put forward this atomic model to explain the electron arrangement
patterns for single-electron species like hydrogen. It only demonstrated the
coarse structure of the atom. But it failed to explain the atomic structure of
multi-electron atoms.
Later, the
quantum mechanics predicted that the gross structure of hydrogen line spectra
is due to non-relativistic spinless electrons. The Bohr model of the atom
explained that the electron without spin orbits the nucleus at lower speeds
than the velocity of light. Additionally, the principal quantum number n
defines these gross structure energy levels. Hence, it counted one spectral
line for every electron transition.
The
hydrogen fine structure is the splitting of spectral lines of the hydrogen atom
due to the interaction of electron spin with the magnetic field generated by
the electron revolution around the atomic nucleus. It is a correction to the
non-relativistic Schrodinger equation relativistically. The splitting of
spectral lines proved the relativistic and electron spin effects result in the
decadence of the energy levels.
In 1916,
Arnold Sommerfeld explained the splitting of hydrogen spectral lines with
elliptical stationary orbits. And he introduced a dimensionless constant α to
account for the energy difference between the gross and fine structure
predictions.
Additional reference:
What are the four main postulates of the Bohr atomic model?
An infographic depicting the postulates of the Bohr atomic model?
Spin-orbit coupling
The electrically
charged spinning electron acts as a magnetic dipole with equal and opposite
magnetic poles in the atom. When the magnetic moment produced by electron spin
angular momentum interacts with the magnetic field of the electron's orbital
motion is called spin-orbit interaction or spin-orbit coupling.
It is a
relativistic interaction of electron spin with orbital angular momentum. It
causes the splitting of electron energy levels into their subdivisions with an
energy partition equal to the total electron energy. The electron transitions
to these sublevels give additional spectral lines due to different transitional
energies.
Hence, the
spin-orbit coupling is a piece of evidence for the splitting of the main
spectral line into two or more ancillary lines. In other words, the interaction
of the spin angular momentum of an electron with its orbital angular momentum
gives a resultant magnetic field. This effective magnetic field is known as
electron spin-orbital angular momentum.
 |
| Splitting of hydrogen spectral line |
The
coupling of the magnetic field generated by the orbiting electron around the
nucleus with quantum mechanical spin produces fine structures of the hydrogen
spectrum. So, the hydrogen fine structures occur due to the coupling of spin
and orbital angular momenta.
Consequently,
the total angular momentum quantum number is the sum of spin and orbital
angular momentum quantum numbers.
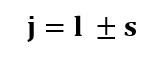 |
| The formula for total angular momentum quantum number |
Where,
j = total
angular momentum quantum number
l = orbital
momentum quantum number
s= spin
momentum quantum number
The value
of l designates the different energy levels of the atom. For example- l=0 we
have s-orbit and l=1 we have p-orbit, l=2 we have d-orbit, and so on. The value
of j is maximum for parallel spin and orbit momentum quantum numbers.
The neutral
particles also show spin-orbit coupling because of the existence of both spin
and orbital angular momenta. In semiconductors, the spin-orbit interaction of
electrons has huge technical advantages.
Additional reference:
A visual description for the angular momentum of the atom
What is the atomic orbital of an atom?
The electron spin
The
electron spin is an intrinsic property of the electron. All the fundamental
particles of matter have a spin of 1/2. The spin number defines the number of
symmetrical facets an electron can have in one complete rotation to reach the
starting point.
We know the
path of an electron around the nucleus is circular. So, the electron takes two
rotational turns to reach its initial point where it started. Therefore the
electron spin is equal to 1/2.
s=1/2
In 1925,
Samuel Abraham Goudsmit and George Eugene Uhlenbeck explained the internal
spinning motion of the electron. And they interpreted that the electron spin
angular momentum is one-half the orbital angular momentum quantum. The two
possible spin angular momentum values for the electron are +ħ/2 or –ħ/2.
The
quantization of the orientation of angular momentum was confirmed by the German
physicists Otto Stern and Walther Gerlach in 1921 with the silver beam
experiment.
Additional reference:
What do you know about energy quantization?
An image depicting the energy quantization of the atom
Stern and Walther Gerlach silver beam experiment- evidence for quantized electron angular momentum
The
stern-Gerlach experiment demonstrated the quantization of spatial orientation
of angular momentum. A beam of neutral silver atoms passed between the poles of
a non-uniform magnetic field. And they observed a deflection in the straight
path of the electron before they stuck the detector film. To elaborate, half of
the silver atoms point toward one direction, and the rest half the silver atoms
point in the opposite direction. It implies the silver atoms act as magnetic
dipoles in the external magnetic field due to the magnetic field gradient that
results in their deflection. In a non-uniform magnetic field, the force exerted
on one end of the dipole is greater than the other end dipole force. The net
force results from the two opposite magnetic dipoles deflecting the particle’s
trajectory.
 |
| The Stern-Gerlach experiment |
The
observations of the detector screen reveal the particle's deflection either up
or down by a specific amount. The discrete points of silver atoms accumulate
owing to the quantized electron spins.
The silver
atom with one unpaired electron in its valence shell spins about its axis. The
unpaired silver electron can spin either in the clockwise or anti-clockwise
direction. Thus, the electron spin direction creates two possible values as +½
and -½. Consequently, the electron spin creates a small magnetic field that
acts like a tiny bar magnet.
The Stern
and Gerlach experiment for electrons blends the trajectory in a circle due to
Lorentz's force. The electron spin deflects either up or down along the
vertical axis with the direction of the magnetic moment. The spin angular momentum
values of electrons measured along any axis are +ħ/2 and –ħ/2. They are also
known as the intrinsic angular momentum of the spinning electron.
 |
| The electron spin orientation |
The
spinning magnetic dipole of an electron with magnetic moment μ and the magnetic
field strength B experiences a torque that rotates it.
 |
| Torque of spinning magnetic dipole |
An electron
with +½ magnetic spin momentum experiences a torque in the magnetic field that
rotates it in one direction. Similarly, the electron with -½ magnetic spin
momentum experiences a torque that rotates it in the opposite direction. Hence,
the potential energy of the magnetic dipole in the magnetic field is the amount
of work done in rotating the dipole.
 |
| The potential energy of magnetic dipole in the magnetic field |
When the
magnetic moment vector is parallel to the magnetic field, it is the position of
the magnetic dipole aligning itself.
Overview of hydrogen fine structure
As a matter
of fact, hydrogen is an alkali metal with a single electron in the 1S-orbit.
Let us imagine the absorption of energy transmitting the hydrogen electron from
1S to 2P level. The electron motion is associated with the orbital quantum
number (l) and the spin quantum number (s). Hence, the total angular moment
quantum number (j) can be expressed as below;
 |
| The total angular moment quantum number for the electron |
When the electron is in 1S-orbit, its spin quantum number values are +1/2 and -1/2 depending upon the direction of the magnetic moment. And the angular momentum quantum number value for S-orbit is zero. So, the total angular momentum quantum number (j) for the 1S-orbit electron is ±1/2.
 |
| The total angular moment quantum number for 1S-orbit electron |
Due to the
absence of spin-orbit coupling in 1S-orbit, splitting does not take place in
electron energy levels. So, the 1S-orbit has a single energy level.
In 2P-orbit, the spin-orbit interaction breaks the main energy level into its components. So, we observe two sub-energy levels for the electron in the 2P-orbit. The spin angular momentum quantum number values for the electron are +1/2 and -1/2. Additionally, the orbital angular momentum quantum number value for P-subshell is 1. The total angular momentum quantum number values are 1/2 and 3/2.
 |
| The total angular moment quantum number for 2P-orbit |
The
hydrogen electron transition from 1S-orbit to 2P-orbit gives spectral lines
doublet as it involves the two 2P-orbit energy sub-states in the electron
transition. The spectral line doublet is a pair of two closely spaced spectral
lines with a slight variation in their wavelengths.
 |
| The effect of magnetic field on the hydrogen spectral emission lines |
The alkali
metal atoms with 1S-electron in their valence shell give spectral line doublet
in the presence of the magnetic field. But, the alkaline earth metals with two
1S-electrons in their valence shell give spectral line triplet due to
spin-orbit interaction. In other words, the number of fine structures increases
with an increase in the stable state configurations in the atom. For this
reason, Lithium with atomic number three (i.e., n=3) may not be resolved by the
average spectroscope. Whereas the Rubidium with atomic number 37 has widely
separated spectral emissions and can be observed with a normal spectroscope.
Additional reference:
What are the six series of the hydrogen spectrum?
A visual explanation showing the hydrogen fine structures
More about the hydrogen fine structures
We know the
hydrogen spectrum consists of six spectral series. The names of those six spectral series of hydrogen are;
- Lyman series
- Balmer series
- Paschen series
- Brackett series
- Pfund series
- Humphreys series
An interesting trick to remember all the six series of the hydrogen spectrum easily
The
hydrogen atom has a single electron in the 1S-orbit. When the hydrogen atom
absorbs energy from the electric current in the discharge tube, it causes the
excitation of hydrogen electron from the ground state to the higher energy
orbit. After being unstable, the excited electron returns to its initial lower
energy state with the emission of photons of suitable wavelengths. When the
emitted photons fall on the detector film, it produces the six series of the hydrogen spectrum.
In the magnetic field, the spectral lines of hydrogen undergo splitting and
create fine structures. Among them, the fine structures of Lyman alpha and
hydrogen alpha are most commonly studied based on their significance in
astronomy.
An engaging image explaining the series of hydrogen spectrum.
Additional reference:
A PowerPoint presentation on the hydrogen spectral series?
An infographic on the Balmer series of the hydrogen spectrum
Lyman alpha
Lyman alpha
spectral line results in the hydrogen spectrum during the electron transition
from the second energy level to the first orbit of the hydrogen atom. It is the
most intense spectral emission in the ultraviolet region of the Lyman series
that occurs at a wavelength of 121.5 nm. The Lyman alpha spectral line splits
to give a pair of spectral lines with a slight variation in their wavelengths
due to the spin-orbit interaction.
An infographic explaining the wavelength a wave
As discussed earlier, the transition of an electron from 1S-orbit to 2P-orbit gives a spectral line doublet in the presence of the magnetic field. The Lyman alpha doublet consists of closely spaced two spectral emission lines at wavelengths of about 121.5668 nm and 121.5674 nm. And they are symbolized as Ly-α3/2 and Ly-α1/2 having j values 3/2 and 1/2, where j is the total angular momentum of the electron.
 |
| The Lyman alpha doublet picture |
The figure
shows a longer arrow for j=3/2 during the transition from 1S-orbit to 2P-orbit.
It indicates a large energy gap between the two stationary sub-shells for
Ly-α3/2 as compared with Ly-α1/2. It realizes Ly-α3/2 is high energy transition
than Ly-α1/2. Hence, Ly-α3/2 spectral emission occurs at a slightly shorter
wavelength than Ly-α1/2. From this conclusion, we can remind of the quantum
theory of radiation for the inversely proportional relationship between the
energy of the photon and the wavelengths of emitted light radiation.
Additional reference:
A beautiful infographic on the Lyman series of the hydrogen spectrum?
Hydrogen-alpha
Hydrogen-alpha
is the shortest spectral emission in terms of energy in the Balmer series of
the hydrogen spectrum. A bright red colored spectral emission at a wavelength
of about 656.28 nm in the hydrogen spectrum is nothing but the hydrogen alpha spectral emission. And the hydrogen electron transition from the principal
quantum number n=3 to n=2 gives this spectral emission in the visible region of
the hydrogen spectrum.
The hydrogen alpha emission line image posted on Apr 1, 2022 on @chemistrylearners Instagram page
The magnetic field generated due to the
coupling interaction of the spin and the orbital angular momentum of the
hydrogen electron during its shift from the 3S-orbit to the 2P-orbit causes the
hydrogen-alpha spectral line splitting. The 2P-orbit splits by the magnetic
field into the sub-energy states doublet with slightly varying energies. Hence,
the electron transition to these modified sub-energy states gives two closely
spaced spectral emission lines with a slight difference in their wavelengths.
.png) |
| The hydrogen alpha spectral line splitting |
According
to Bohr’s energy equation, the energy difference between the second and third
orbits of the hydrogen atom is equal to 7.5 eV. The hydrogen-alpha spectral
line in the absence of a magnetic field occurs at a wavelength of 656.2 nm. In
the magnetic field, the H-α line undergoes splitting into two closely spaced
spectral emission lines with a wavelength variation of about 0.016 nm.
 |
| The formula calculates the energy difference in the hydrogen alpha spectral emission |
Since the
start of the spin-orbit coupling interaction concept, we have constantly
discussed the splitting of spectral lines by the electron transitions between
the S and P-orbits of the atom. So, you might get a question like the one
below;
Additional reference:
An infographic explaining the hydrogen alpha spectral line
What is the Balmer series of hydrogen spectrum-a PowerPoint presentation
An infographic on the comparative explanation of the Balmer series
An exclusive blog post for the Balmer series of the hydrogen spectrum
Did the electron transition only between S and P orbits give spectral splitting?
It is
absurd. The atomic structure is not limited to S and P sub-shells. With the
increase in atomic number, the number of stationary orbits for the atom
increases that it may even include d and f sub-shells. The stationary orbits
with non-zero angular momentum quantum numbers give magnetic interaction by the
spin-orbit coupling. Let us have a look at the value of l for different
sub-shells like s, p, d, and f at once.
The value
of l for S-orbit is zero. So, it cannot split into its constituent sub-states.
S-orbit has a single energy level irrespective of the value of the principal
quantum number. And the l value for p,d,f sub-shells are 1,2,3 respectively.
Hence, all these three sub-levels participate in splitting. The electron
transition from the S-orbit to any of the three sub-states gives spectral line
splitting regardless of the n value.
So, the
spectral line splitting is not limited to S and P orbits.
Additional reference:
Why the Balmer series is visible?
The visual representation of the hydrogen alpha emission line
The differences between the Lyman alpha and hydrogen alpha fine structures
So far, we
have discussed the Lyman alpha and hydrogen alpha splitting patterns. Now let
us discuss the difference between them.
- The Lyman alpha splitting takes place during the electron transition between the second and first stationary orbits of the hydrogen atom. Similarly, the hydrogen alpha spectral splitting occurs by the electron transition between the third and second stationary orbits.
- The Lyman alpha spectral emission splitting happens by 1S-orbit and 2P-orbit interaction in the magnetic field. But, the hydrogen alpha splitting involves the 3S and 2P orbits spin and angular momentum quantum numbers interaction.
- The energy difference between the two energy levels for Lyman alpha splitting is 10.2 eV. Whereas for hydrogen alpha splitting, the ΔE value is 7.5 eV.
- The Lyman alpha spectral line occurs in the ultraviolet region of the hydrogen spectrum with a wavelength of 121.5 nm. In the same way, the hydrogen alpha spectral line comes in the visible zone of the hydrogen spectrum with a wavelength of about 656.2 nm.
- The wavelength difference for the fines structures of Lyman alpha is 0.0006 nm. And in the case of hydrogen-alpha, it is about 0.016 nm.
Apart from
the differences, they both have the most common applications in astronomy. They
used to identify quasars, unknown astronomical bodies in the universe. They are
also helpful in calculating redshifts.
 |
| The difference between the fine structures of Lyman alpha and hydrogen alpha |
Lorentz force
The Dutch
physicist Hendrik Antoon Lorentz was born in Arnhem, Neth, on July 18, 1853. He
shared Nobel Prize with Pieter Zeeman in 1902 for the electromagnetic theory of
light radiations.
Lorentz
interpreted the relationship between electricity, magnetism, and light with his
theory. And it was the refined explication of James C. Maxwell's
electromagnetic theory.
Maxwell
found that the oscillations of electric charges produce electromagnetic
radiation. But, he did not clarify the particular charged particle that
generated the light radiation.
Later,
Lorentz expanded Maxwell's findings to atoms. And he clarified that since the
electric current is composed of charged particles, the oscillations of these
charged particles are accurately the electron oscillations. And he assumed that
these electron oscillations are responsible for the emission of light
radiations in the atom.
He added
that the electron oscillation was affected by the subsisting magnetic field of
the atom that in turn influences the wavelength of emitted light radiation.
Lorentz's
investigations were unsuccessful in overcoming the drawbacks of the
Michelson-Morely experiment. Therefore, he introduced the local time theory to
explain the different time rates of the various locations. He suggested that
moving bodies contract their direction of motion while approaching the velocity
of light.
The two
parametrized vectors, E for the electric field and B for the magnetic field,
defined the electromagnetic force acting on a point charge q in the functional
form that moves with a velocity v at a given point and time.
And the
electromagnetic force is a function of the charge q and its velocity v.
 |
| Lorentz force formula |
In fact,
the test charge q would generate its own finite electric and magnetic fields
that alter its electromagnetic force.
If the
charge experiences acceleration, it loses its kinetic energy by emitting light
radiation. And the emitted light is forced into a curved trajectory.
 |
| Trajectory of a particle under magnetic field |
Zeeman Effect
The Dutch
physicist Pieter Zeeman was born in Zonnemaire, Neth, on May 25, 1865. Zeeman
completed his studies at the University of Leiden. And he rendered his service
as a physics lecturer at Leiden in 1890. In 1900, Zeeman became a physics
professor at the University of Amsterdam. And in 1908, he became the director
of the Physical Institute, where he served till his death.
As
mentioned earlier, he shared Nobel Prize for physics with Lorentz in 1902 for
his discovery of the Zeeman Effect.
Zeeman
elucidated the effect of a magnetic field on a source of light. He explained
the cause for the splitting of emitted light radiations of the atomic spectra.
The
interaction of the light source of the atom with the external magnetic field to
split the spectral lines into their fine structures was named the Zeeman
Effect. While experimenting on the yellow D-lines of sodium, Zeeman first
observed it in 1896.
When the
sodium emission lines passed through the external magnetic field, the
broadening of the sodium spectral line took place. And it showed the splitting
of distinct spectral lines into 15 components.
From the
experimental evidence, he concluded that the electron movement from one
discrete energy level to the other causes the emissions of photons of definite
wavelengths, which give spectral lines in the atomic spectra. A quantity named
angular momentum characterizes each distinct energy level of the atom. In the
absence of a magnetic field, there is no splitting in the atomic energy levels.
Hence, they exhibit a single spectral line even under a high-resolution
spectrometer.
But in the
external magnetic field, these stationary energy levels of atoms split into
their sub-states of slightly different energies. The electron transitions to
these sub-states of energy give more spectral lines than expected. In other
terms, it is referred to as the splitting of the main spectral line into its
components in the external magnetic field. The additional spectral lines are
also known as fine structures. The phenomenon is known as the Zeeman effect.
Applications of Zeeman effect
We all know
the splitting of spectral lines served as an efficient method to identify the
magnetic field strength in the chemical elements. In addition to this, the
Zeeman effect helped in the following ways.
The Zeeman
effect helped scientists specify the atomic energy levels in terms of angular
momenta.
It
presented an effective method to study the atomic properties concerning atomic
nuclei like electron paramagnetic resonance.
It calculated
the magnetic effects of spectral lines effectively. Therefore, it is used to
estimate the magnetic fields of celestial bodies in astronomy.
Overview of the Zeeman effect
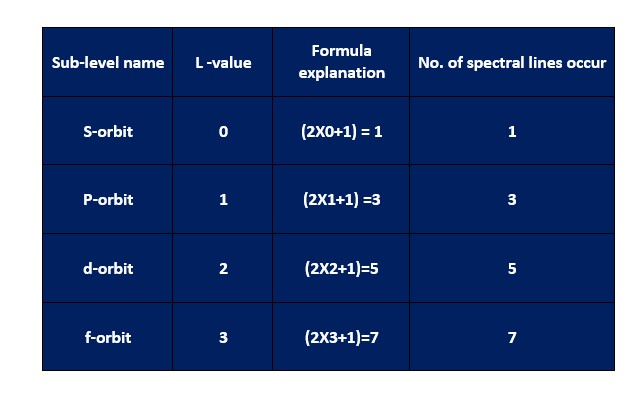
Zeeman
suggested a mathematical formula to calculate the number of fine structures in
spectral line splitting by the external magnetic field.
 |
| Zeeman formula |
Where,
L is the orbital angular momentum quantum number and its value can be a non-negative integer.
The
following table describes the value of the orbital angular momentum quantum
number for various atomic levels.
 |
| Table for calculation of number of spectral lines by the Zeeman formula |
The above
table explains the number of spectral lines obtained by the interaction of the
external magnetic field with the light source. And the number of hydrogen fine
structures due to the interference of the external magnetic field in the
hydrogen electron transition from S-orbit to P-orbit is three.
The S-orbit has zero orbital angular momentum
number. And it does not show spectral line splitting even in the external
magnetic field. Hence, it has a single energy level.
But, for
P-orbit, the value of l=1. Consequently, in an external magnetic field, it
splits into three sub-energy states that give three hydrogen fine structures by
the spectral line splitting.
 |
| The Zeeman effect splitting pattern |
Regardless
of the principal quantum number value, the hydrogen electron transition
involves the similar S and P atomic sub-levels in the Lyman alpha or
hydrogen-alpha. Hence, the previous explanation justifies these spectral
emissions.
The
spectral lines which show the Zeeman Effect exhibits polarization. This
phenomenon affects the direction of the vibrating electromagnetic field. It
influences the appearance of the spectral emission lines.
A sunspot
emits three spectral lines by the influence of a strong magnetic field due to
the Zeeman effect. Only two among the three spectral lines are visible from the
top view due to the polarization effect. The suppression in the spectral line
visibility gets over by changing the observer's direction of view. With the
change of angle of observation, a weak third spectral line appears.
Why is the difference in the number of fine structures observed for the spin-orbit coupling and Zeeman effect?
The concept behind the two mentioned phenomena
is the same. The hydrogen electron, while absorbing energy, undergoes a
transition between the discrete transition states, and the process goes on with
the emission of photons of varying frequencies that result in spectral lines in
the spectrum.
The
interference of the magnetic field with the light source causes spectral line
splitting that gives rise to fine structures.
In
spin-orbit coupling, the electron spin interacts with the orbital angular
momentum. Thus, it creates an internal magnetic field. And the generated
magnetic field splits the main spectral line into its fine structures.
Similarly,
When passed through the poles of an external magnetic field, the light source
shows the spectral line splitting known as the Zeeman effect.
For
example- The electron transition from S-orbit to P-orbit gives two spectral
lines in the spin-orbit coupling. Besides, the same electron transitions result
in three spectral lines by the Zeeman effect. It solely depends on the nature
of the magnetic field that interacts with the emitted light. Hence, we observe
a difference in the number of spectral lines in both phenomena.
Additional reference:
A beautiful visual interpretation for the Zeeman effect
Paschen back effect:
This effect
is observed when the emitted light source passes through the strong magnetic
field. The hydrogen atom on the absorption of energy shows an electron
transition that emits photons of definite wavelengths. These emitted light
radiations show spectral lines when entrapped on the detector film. The emitted
photons that pass through a strong external magnetic field poles show a
different pattern of spectral splitting when analyzed by the high-resolution
spectrometer. This spectral splitting is due to the coupling of the external
magnetic field with the light source. And it is known as the Paschen back
effect. Two German physicists, Paschen and Ernst Back in 1921, observed this
effect for the first time.
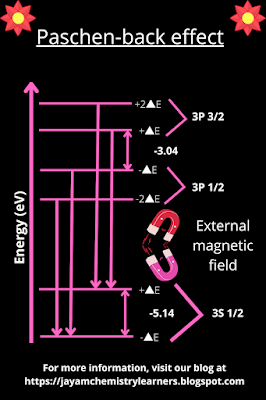 |
| The explanation for the electron transitions in the sodium atom by the Paschen back effect |
The energy
difference is expressed as a multiple of Bohr magneton (μB)
 |
| The energy difference formula for Paschen back effect |
Sodium is
used to interpret this model for convenience. The spectral line splitting in
the sodium metal depicts that the transition of an electron from 3S-orbit to
sub-energy levels of 3P-orbit gives different kinds of spectral emission lines
than the Zeeman effect. The resulting spectrum shows a triplet, with the
central spectral line having double the intensity as the remaining two spectral
emission lines.
The
Paschen-back effect conditions are in the sun for Lithium spectra. It does not
have much astronomical significance.
Additional reference:
A visual representation for the Paschen back effect for easy understanding
What is the Paschen series of the hydrogen spectrum?
The differences between the Zeeman effect and the Paschen back effect:
So far, we
have discussed the effect of the magnetic field on spectral line splitting. We
discussed the two kinds of magnetic field interactions. They are external
magnetic field and internal magnetic field interactions.
Based on
the magnetic strength, the magnetic field is of two types.
- Weak magnetic field
- Strong magnetic field
Case-I: Weak magnetic field (Zeeman effect)
The
interaction of spin and orbital angular momentum is stronger than the external
magnetic field coupling in the case of the weak magnetic field. In such
scenarios, the Spin-orbit effect is a dominant factor that causes spectral line
splitting. The coupling of orbital angular momentum quantum number with
electron spin angular momentum generates spin-orbit coupling. The induced
internal magnetic field splits the spectral line into its components. Hence, the
source of light that passes through the poles of the weak magnetic field
diverges the spectral emission line due to spin-orbit interaction. The Dutch
physicist Pieter Zeeman in the year 1896, observed this splitting on the yellow
D-lines of sodium. And it is known as the Zeeman effect.
 |
| The demonstration of magnetic field in the Zeeman effect |
The amount
of splitting is less when compared with the energy difference between the
unperturbed levels in the weak magnetic fields.
Both the
orbital angular momentum vector and the electron spin vector jointly contribute
to the direction of the magnetic field.
The total
angular momentum quantum number is the sum of the two vector quantities, i.e.,
orbital angular momentum quantum number and electron spin quantum number.
Case-II: Strong magnetic field (Paschen back effect)
The
presence of the large magnetic field disrupts the interaction of the spin and
orbital angular momentum quantum numbers of the atom. The source of light that
passes through the poles of a strong magnetic field shows a different splitting
pattern due to the interaction with an external magnetic field rather than
spin-orbit interaction. The decoupled spin-orbit interaction in the
sufficiently large external magnetic field leading to the spectral line
splitting with more fine structures is known as Paschen back effect. Two German
physicists, Paschen and Ernst Back in 1921, observed this effect for the first
time.
 |
| The demonstration of magnetic field in the Paschen back effect |
The amount
of splitting is considerable when compared with the energy difference between
the unperturbed levels.
The orbital
angular momentum vector and the electron spin vector individually define the
direction of the magnetic field.
The total
angular momentum quantum number is no longer a constant for particle motion.
Additional reference:
An infographic on the differences between the Zeeman effect and the Paschen back effect
Question and answers on the hydrogen fine structure topic:
Why did Bohr's atomic model fail to explain the fine structures of the hydrogen atom?
Bohr atomic
model successfully explained the electronic arrangement of the atom with
quantized electron orbits. Still, it could not explain the thin and narrow
splitting structures of the hydrogen spectrum. The following reasons might be
an accurate explanation for this limitation.
- Non-relativistic spin less electron motion could not produce a magnetic field in the atom.
- The Bohr model of the atom explained that the electron without spin orbits the nucleus at lower speeds than the velocity of light.
- Additionally, the principal quantum number n defines these gross structure energy levels. Hence, it counted one spectral line for every electron transition.
All these
misinterpretations result in the gross spectral structures in the hydrogen
spectrum.
For an interesting infographic on Bohr model Vs. fine structures of hydrogen atom, refer here.
Why does the splitting structures of the atom named fine structures?
The
spectrum observations with an ordinary spectrometer cannot reveal the thin,
narrow, delicate structures of a spectral line resulting from the electron
transition. But a high refined spectrometer can divulge them. The single
spectral line of an atomic spectrum is a collection of silky-splitting
structures when hit by a magnetic or electric field. These unequal energy lines
were named fine structures based on their appearance after the invention of
high refined spectrometers.
Did the atomic number affect the splitting of spectral lines?
Yes, the
number of fine structures increases with an increase in the stable state
configurations in the atom.
The alkali
metal atoms with one 1S-electron in their valence shell give spectral line
doublet in the presence of the magnetic field. But, the alkaline earth metals
with two 1S-electrons in their valence shell give spectral line triplet due to
spin-orbit interaction.
An average
spectrometer cannot show the fine structure of the Lithium under ordinary
conditions. But, the same spectroscopic observation circumstances can divulge
the widely spread spectral emissions of Rubidium metal due to its high atomic
number and consequently more stable state configurations.
Who explained the fine structures of the hydrogen spectrum with relativistic corrections?
Arnold
Sommerfeld, the German physicist who pioneered the atomic and quantum physics
evolutions and guided many chemistry and physics Nobel Prize winners to achieve
their scientific goals, had explained the fine structures of the hydrogen
spectrum with relativistic corrections.
Neil Bohr's
model of atom explicated the line spectrum of hydrogen with non-relativistic
electron motions. But, these assumptions failed in interpreting the fine
spectral structures.
Sommerfeld
recognized this opportunity to add relativistic corrections to hydrogen
spectral emissions. And his fine structure constant α elucidated the gap
between the gross Bohr spectral structures and the fine structures of spectral
splitting.
 |
| Fine structure constant representation |
To point
out the unequal energy distributions of spectral splitting, he introduced the
second quantum number (azimuthal quantum number) to explain the orbital angular
momentum of the electron.
The
azimuthal quantum number invention and relativistic electron motion corrections
solved the fine structure limitation.
Conclusion:
We hope that this blog post is helpful to you. And what else
do you know about the fine structures of the hydrogen atom? Kindly share your knowledge with us in
the comments below. And also, you can visit our blog
https://jayamchemistrylearners.blogspot.com/ regularly for more engaging topics
of chemistry. We add new posts regularly to our blog. To not miss any updates,
kindly follow us. We will notify you immediately. Most importantly, you can ask
your questions on the hydrogen fine structures topic in the post comment section and
also on our Instagram page. We are happy to hear from you and will answer you.
For more fascinating chemistry topic visuals, please visit
and follow our Instagram page @chemistrylearners and Pinterest page
@kameswariservices.
