The six series of the hydrogen spectrum-chemistry learners
Hydrogen spectral series
Hydrogen spectral series introduction:
 |
| The hydrogen spectral series visual |
What is the spectral series of the hydrogen atom?
 |
| The dissociation of hydrogen molecule into its atoms |
 |
| The schematic representation of the discharge tube |
History of the hydrogen spectral line series
 |
| The spectrum of sunlight |
 |
| The Balmer formula |
 |
| The Rydberg formula |
Name the series of the hydrogen spectrum
- Lyman series
- Balmer series
- Paschen series
- Brackett series
- Pfund series
- Humphreys series
 |
| The six series of the hydrogen spectrum |
- The electron movement from higher transition states of n>1 to the first static state (n=1) is the Lyman series. Theodore Lyman, in 1915, found this series in the ultraviolet region of the electromagnetic spectrum.
- Johann Jakob Balmer, in 1885, found a sequence of spectral emissions for the electron transitions from stationary states that are n>2 to the second orbicular configuration of the hydrogen atom. It is known as the Balmer series.
- In 1896, Friedrich Paschen named a group of spectral lines as the Paschen series that appeared in the infrared region of the electromagnetic spectrum. It involves the electron transference to stagnant states n≥3 from the third energy level.
- In 1922, Frederick Sumner Brackett found a bunch of spectral emissions in the n≥4 transitions of the electron that occur in the infrared region.
- The electron movement from n=5 state to higher excited states with wavelengths of spectral lines above 700 nm is none other than the Pfund series, August Herman Pfund found them in 1925.
- In 1953, Curtis J. Humphreys found a sequence of spectral lines in the far infrared region of the electromagnetic spectrum. It is known as the Humphreys series. Moreover, it involves the stationary states that are n≥6 to lower energy state n=6.
 |
| The hydrogen spectral series table |
Regions of the six series of the hydrogen spectrum
 |
| Regions of the hydrogen spectral series |
Q.1. Why does the line spectrum of hydrogen's lines becomes closer as the frequency increases?
 |
| Energy-Frequency relationship by the quantum theory |
According to the quantum theory of radiation, the energy
difference between the stationary levels varies directly with the frequency of
the emitted light radiation. It implies the frequency of the emitted photon is
higher for electron transitions involving higher transition states.
For example- The electron transition from second to third
energy states of the hydrogen atom gives a spectral line in the visible region
with a frequency corresponding to the energy difference between those two
energy levels.
Again, the electron transitions from second to fourth static
orbicular states of the hydrogen atom give a spectral emission at a higher
frequency than the previous one. It shows a large energy gap between the two
stationary states involved in electron transition.
Moreover, the stationary orbits are not equally spaced. They
are more close together at higher energy levels. So, the electron transitions
involving these closely spaced orbits give spectral lines packed together.
Lyman series, the broad series of the hydrogen spectrum,
gives thick spectral emissions towards its end. Likewise, the Balmer and
Paschen series are more compact when compared with the Lyman series. We observe
a thick spectral line region at the series limit of every series. It is the
position where the next series starts.
 |
| Frequency variation in the hydrogen spectral series |
As a final note, the dense spectral emissions towards the right end of the hydrogen spectrum imply higher photon frequencies.
Hydrogen spectrum series formula
We have already discussed the Johannes Rydberg formula as
the extension of the Balmer formula. The Rydberg formula helps to calculate the wavelengths of all spectral lines that occur in the hydrogen spectrum with the
help of an empirical fitting parameter known as the Rydberg constant.
 |
| The Rydberg formula |
Where,
λ = wavelength of the emitted electromagnetic radiation
n1 = lower energy level of the electron
transition
n2 = higher energy level of the electron
transition
R= Rydberg constant with value equal to 109678 cm-1
Trick to remember hydrogen spectral series easily
As promised earlier, I will now discuss the trick to
remember all the six series of the hydrogen spectrum with a simple, catchy
sentence. Are you ready to have a look at it?
Yeah, to memorize the hydrogen spectrum series effortlessly,
recall this sentence.
Lovely Balloons Pair
Brought Pforth Humphrey
Lovely - Lyman series
Balloons - Balmer series
Pair - Paschen series
Brought - Brackett series
Pforth - Pfund series
Humphrey - Humphreys series
 |
| Trick to remember the hydrogen spectral series easily |
The six series of the hydrogen spectrum
We have a general idea about the different series of the
hydrogen spectrum. The six sequences of the hydrogen emission spectrum
correspond to the discontinuous spectral line emissions due to quantized
electron energy levels of the hydrogen atom explained by Niels Bohr. The
transition of electrons between the two stationary orbits results in the
erratic emission of light energy at specific frequencies. It results in the
individually distinct spectral lines in the atomic spectrum of hydrogen. Hence
the other name for it is hydrogen line spectrum.
Without further delay, let us discuss the hydrogen spectral
line series briefly.
Lyman series
Forewords
This series of spectral lines were observed during electron transition from higher stationary orbits to the first orbit of the hydrogen atom and named after the discoverer Theodore Lyman. And it occurs in the ultraviolet region of the electromagnetic spectrum.
 |
| The Lyman series formula |
nf is
higher energy orbit. It’s value can be 2,3,4,…..,∞
RH is called the Rydberg constant for the
hydrogen atom
λ is the wavelength of emitted spectral line
Scientist life
The U.S. Physicist and spectroscopist Theodore Lyman IV was
born on November 23, 1874, in Massachusetts in Boston. He completed his Ph.D.
from Harvard University in physics and rendered his service as a Physics
professor at Harvard University.
He researched light radiations of shorter wavelengths,
particularly ultraviolet radiations and their properties. It made him discover
the first line in the ultraviolet region of the Lyman series in 1906. By
extending his hydrogen spectrum studies, he found the rest of the lines in the
Lyman series from 1906 to 1914.
In addition to that, he studied the diffraction gratings
phenomenon. With his contributions to physics, he was awarded the Franklin
Institute's Elliott Cresson Medal in 1931.
Overview
The electron transitions from n ≥2 to n=1 result in the
emergence of a sequence of spectral lines in the Lyman series of the hydrogen
spectrum. Consequently, the n1 and n2 values of the Lyman series vary from 1 to
infinity. The Greek letters represent these electron transitions as
Lyman-alpha, Lyman-beta, Lyman-gamma, etc.
 |
| The Lyman series diagram |
 |
| Lyman series table |
Likewise, the longest and shortest wavelengths of the Lyman
series are 121 nm and 91 nm. Hence, the limits of the Lyman series are 91nm and
121nm.
 |
| Calculation of wavelengths in the Lyman series |
The detailed wavelength data of the Lyman series is in the following table.
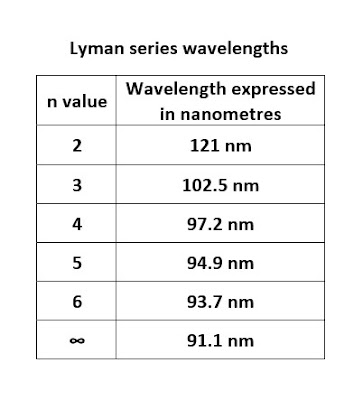 |
| The Lyman series wavelengths table |
The above table confirms that the maximum wavelength for the Lyman series is 121 nm and the minimum wavelength is 91 nm.
Lyman-alpha
Lyman observed the most intense spectral line at 121 nm during the electron journey from n=2 to n=1 and named Lyman Alpha. Because, on absorbing energy from an external source, a bulk proportion of hydrogen atoms move from the ground state to n=2 state. It results in a strong emission line at 121 nm.
Additional reference:
What is the importance of the Lyman-alpha line?
Why does the Lyman series lie in the ultraviolet region?
The Lyman series lies in the UV region because all the spectral lines of this series have wavelengths below 400nm. And the wavelength of lines obtained below 400 nm falls in the ultraviolet part of the electromagnetic spectrum.
Application of the Lyman series
Formation of Ozone
The oxygen molecules present in the upper stratosphere absorb the Lyman alpha emissions from ultraviolet rays. Then, they dissociate into oxygen atoms. It results in the formation of ozone from oxygen atoms and molecules.
 |
| Formation of Ozone |
Balmer series
Forewords
This series of spectral lines were observed during electron
transition from higher stationary orbits to the second orbit of the hydrogen
atom and named after the discoverer Johann Jakob Balmer. And it occurs in the visible region of the electromagnetic spectrum.
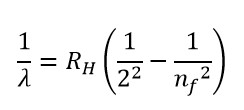 |
| The Balmer series formula |
nf is
higher energy orbit. It’s value can be 3,4,5,…..,∞
RH is called the Rydberg constant for the
hydrogen atom
λ is the wavelength of emitted spectral line
Scientist life
Johann Jakob Balmer was born in Lausen, Switzerland on, 1
May 1825. From childhood, he had a good command of Mathematics subject. Balmer
graduated from the University of Karlsruhe and the University of Berlin in
Mathematics. And at the University of Basel, he submitted his thesis on the
cycloid in 1849. He spent his entire as a maths teacher in Basel and delivered
his lectures at the University of Basel.
His great benefaction to astronomy and chemistry is
discovering an empirical formula to estimate the spectral lines in the visible
region of the hydrogen spectrum in 1885. It calculated the spectral line
emissions when the electron alteration from stationary configuration greater
than two to an orbicular state equals two. To do so, he took the radius of the
hydrogen atom (0.529 A0) into account. His formula for determining
the wavelength of the spectral line is as follows:
 |
| The Balmer formula |
Where,
B is Balmer constant with a value equal to 364nm
m is a principal quantum number that equals two
n is an integer such that n>2
He then used his formula to calculate the wavelength of the
spectral line for an electron transition from the energy level equal to 7 to
the second. He succeeded in matching his result with the wavelength of the
spectral line observed by Angstrom at 397nm.
Overview
The electron transitions from n ≥3 to n=2 result in the
emergence of a sequence of spectral lines in the Balmer series of the hydrogen
spectrum. Consequently, the n1 and n2 values of the Balmer series vary from 2
to infinity.
Balmer series of the hydrogen spectrum consists of spectral
lines in the visible and ultraviolet regions. In the visible zone, four
different lines at wavelengths 656nm, 486nm, 434nm, and 410nm correspond to
electron transitions from energy levels such as 3 to 2, 4 to 2, 5 to 2, 6 to 2
gives characteristic red, aqua, blue and violet coloured lines in the spectrum.
And again, in the ultraviolet part, he observed four spectral lines
corresponding to electron transferences from higher stationary states such as 7
to 2, 8 to 2, 9 to 2, and ∞ to 2 at wavelengths 397nm, 388nm, 383nm, and 365nm.
An infographic on comparative explanation on the visible and UV regions of the Balmer series is here.
 |
| The Balmer series diagram |
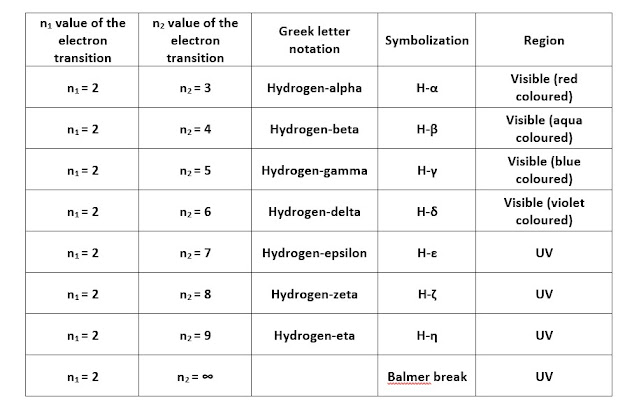 |
| Balmer series table |
Likewise, the longest and shortest wavelengths of the Balmer
series are 656 nm and 365 nm. Hence, the limits of the Balmer series are 656 nm
and 365 nm.
 |
| Calculation of wavelengths in the Balmer series |
The detailed wavelength data of the Balmer series is in the
following table.
 |
| The Balmer series wavelengths table |
The above table confirms that the maximum wavelength for the
Balmer series is 656 nm and the minimum wavelength is 365 nm.
Hydrogen-alpha
At sufficient temperature, Balmer observed the most intense
red spectral line during the electron movement from third to second energy
state in the emission spectrum of hydrogen and named it hydrogen-alpha. It is
one of the conspicuous colors on the earth's crust due to the abundance of
hydrogen.
H-alpha is the deep red colored spectral line in the visible
region of the hydrogen emission spectrum with a wavelength of 656 nm in air.
Additionally, it is the brightest hydrogen line in the hydrogen color spectrum.
It helps the astronomers to estimate the amount of ionized hydrogen content in
the gas clouds.
Which is the only series of hydrogen spectrum with colors?
The Balmer series of the hydrogen spectrum consists of
spectral lines in the visible region with a wavelength between 400-700 nm.
These spectral lines are colored that can be seen with the human eye.
Additional reference
An infographic on the history of the Balmer series
Paschen series
Forewords
This series of spectral lines were observed during electron transition from higher stationary orbits to the third orbit of the hydrogen atom and named after the discoverer Friedrich Paschen. And it occurs in the near infrared region of the electromagnetic spectrum.
 |
| The Paschen series formula |
nf is higher energy orbit. It’s value can be 4,5,6,…..,∞
Scientist life
The German physicist Louis Carl Heinrich Friedrich Paschen
was born on 22 January 1865 in Schwerin. He completed his studies at the
universities of Berlin and Strassburg. After that, he became a professor at
various universities, including the University of Berlin. Moreover, he taught
in Berlin until his death.
In 1908, he observed
a series of spectral lines in the infrared region of the electromagnetic
spectrum. Then, he is popularly known for his discovery of a series in the
hydrogen spectrum. And it is named the Paschen series. Later, he explained the
spectral lines splitting in a high magnetic field known as the Paschen-back
effect.
He was honored with a Rumford medal in 1928 for his
contributions to the knowledge of spectra.
Overview
The electron transitions from n ≥4 to n=3 result in the
emergence of a sequence of spectral lines in the Paschen series of the hydrogenspectrum. Consequently, the n1 and n2 values of the Paschen series vary from 3
to infinity.
 |
| The Paschen series diagram |
Likewise, the longest and shortest wavelengths of the Paschen
series are 1875 nm and 821 nm. Hence, the limits of the Paschen series are
821nm and 1875nm.
 |
| Calculation of wavelength in Paschen series |
The detailed wavelength data of the Paschen series is in the
following table.
 |
| The Paschen series wavelength table |
The above table confirms that the maximum wavelength for the
Paschen series is 1875 nm and the minimum wavelength is 821 nm.
Paschen-delta
Paschen-delta is the spectral line observed at 1005 nm
during the electron journey from n=7 to n=3.
In which region do the Paschen series appear?
The Paschen series lies in the near-infrared region because
all the spectral lines of the series have wavelengths below 1900nm. And the
wavelength of the spectral lines that lie between 700-1900 nm is assumed as the
near-infrared region.
Additional reference:
What is the empirical relation for the spectral lines in the Paschen series?
Brackett series
Forewords
This series of spectral lines were observed during electron
transition from higher stationary orbits to the fourth orbit of the hydrogen
atom and named after the discoverer Frederick Sumner Brackett. And it occurs in
the infrared region of the electromagnetic spectrum.
 |
| The Brackett series formula |
nf is higher energy orbit. It’s value can be 5,6,7,...…,∞
Scientist life
Frederick Sumner Brackett, the American physicist, and
spectroscopist, was born on August 1, 1896, in Claremont, California. After
graduation from Pomona College, he worked as an observer at Mount Wilson
Observatory. Here, he used to observe the Sun's infrared radiations.
He completed his Ph.D. in physics from the Johns Hopkins
University in 1922. In the same year, he discovered a spectral series in the IR
region of the hydrogen spectrum. Then, it was named after him as the
"Brackett series."
He developed a spectrometer containing the world's largest
natural quartz prisms. With its help, he researched toxic substances in body
fluids at the National Institute of Health (NIH) when he rendered his service
as a director.
For his tireless efforts to the scientific world, a lunar
crater was named with his name when he was alive.
Overview
The electron transitions from n ≥5 to n=4 result in the
emergence of a sequence of spectral lines in the Brackett series of thehydrogen spectrum. Consequently, the n1 and n2 values of the Brackett series
vary from 4 to infinity.
 |
| The Brackett series diagram |
Likewise, the longest and shortest wavelengths of the
Brackett series are 4051 nm and 1458 nm. Hence, the limits of the Brackett
series are 4051 nm and 1458 nm.
 |
| Calculation of wavelength in the Brackett series |
The detailed wavelength data of the Brackett series is in
the following table.
 |
| The Brackett series wavelengths table |
The above table confirms that the maximum wavelength for the
Brackett series is 4051 nm and the minimum wavelength is 1458 nm.
Additional reference:
What is the shortest wavelength for the Brackett series?
Pfund series
Forewords
This series of spectral lines were observed during electron
transition from higher stationary orbits to the fifth orbit of the hydrogen
atom and named after the discoverer August Herman Pfund. And it occurs in the far infrared region of
the electromagnetic spectrum.
 |
| The Pfund formula |
nf is higher energy orbit. It’s value can be 6,7,8,…..,∞
Scientist life
The American physicist, August Herman Pfund, was born on
December 28, 1879, in Madison, Wisconsin. He graduated in physics subject from
the University of Wisconsin.
Under Robert W. Wood's advisory, Pfund earned his Ph.D. at
Johns Hopkins University in 1906. He served at Johns Hopkins University as a
full-time professor and chair of the physics department later. In addition to
that, he rendered his service as the president of the Optical Society of
America in 1943.
In 1925, Pfund discovered the fifth series of the hydrogen
spectrum known as the Pfund series. One of his great inventions is the Pfund
telescope to achieve the fixed telescope focal point irrespective of the
telescope line of sight position. His second vital invention was the Pfund sky
compass in 1944, which arose from his research on scattered light polarization
in the sky. It determined the sun's direction in the twilight that helped the
transpolar flights.
For his noted inventions and work in infrared gas analysis, he got Edward Longstreth Medal in 1922 and Frederic Ives Medal in 1939.
Overview
The electron transitions from n ≥6 to n=5 result in the
emergence of a sequence of spectral lines in the Pfund series of the hydrogenspectrum. Consequently, the n1 and n2 values of the Brackett series vary from 5
to infinity.
 |
| The Pfund series diagram |
Likewise, the longest and shortest wavelengths of the Pfund
series are 7460 nm and 2280 nm. Hence, the limits of the Pfund series are 7460
nm and 2280 nm.
 |
| Calculation of wavelength in the Pfund series |
The detailed wavelength data of the Brackett series is in
the following table.
 |
| The Pfund series wavelengths table |
The above table confirms that the maximum wavelength for the
Pfund series is 7460 nm and the minimum wavelength is 2280 nm.
Additional reference:
What do you mean by the series limit?
Humphreys series
Forewords
This series of spectral lines were observed during electron
transition from higher stationary orbits to the sixth orbit of the hydrogen
atom and named after the discoverer Curtis Judson Humphreys. And it occurs in
the far infrared region of the electromagnetic spectrum.
 |
| The Humphreys series formula |
nf is higher energy orbit. It’s value can be 7,8,9,…..,∞
Scientist life
Curtis Judson Humphreys, the American physicist, was born on
17 February 1898 in Alliance, USA. He completed his education at the University
of Michigan. He worked in the Radiometry Section of the U.S. Navy. He was
involved in the Spectroscopic programs of the NBS and U.S. Naval Ordnance
Laboratory. He had set up the atomic wavelength standard in the infrared at the
Corona Lab program.
In 1953, he discovered the sixth hydrogen spectrum series in
the far-infrared region named the Humphreys series. He wrote books such as
"The 29 and 30 electron-system spectra of arsenic and selenium" in
1928 and "First spectra of neon, argon, and xenon 136 in the 1.2–4.0 µm
region" in 1973.
He got the William F. Meggers award in Spectroscopy in 1973.
And he was listed in "World Who's Who in Science" in 1968.
Overview
The electron transitions from n ≥7 to n=6 result in the
emergence of a sequence of spectral lines in the Humphreys series of the
hydrogen spectrum. Consequently, the n1 and n2 values of the Humphreys series
vary from 6 to infinity.
 |
| The Humphreys series diagram |
Likewise, the longest and shortest wavelengths of the
Humphreys series are 12.37 μm and 3.282 μm. Hence, the limits of the Humphreys
series are 12.37 μm and 3.282 μm.
 |
| Calculation of wavelength in the Humphreys series |
The detailed wavelength data of the Humphreys series is in
the following table.
 |
| The Humphreys series wavelengths table |
The above table confirms that the maximum wavelength for the
Humphreys series is 12.3 μm and the minimum wavelength is 3.282 μm.
Higher hydrogen spectrum series (n≥7):
Infinite series of the hydrogen spectrum is possible but are
unnamed. The higher hydrogen spectrum series are expanded and emerge spectral
lines at higher wavelengths. These spectral lines observed at higher energy
levels are faint and correspond to rare atomic events.
The Rydberg formula gives accurate results for the
single-electron systems. But it's deduction is essential to extend it to
spectra of the other elements.
The explanation for the occurrence of an infinite number of
spectral lines in the hydrogen spectrum:
As discussed earlier, the hydrogen spectrum consists of an
infinite number of spectral series that are unnamed. It corresponds to the
boundless stationary orbits ranging from 1 to infinity. The Rydberg formula
helps to measure the wavelengths of those spectral lines precisely.
 |
| The Rydberg formula |
Where,
n1 and n2 corresponds
to higher and lower energy levels of electron transition.
R is called Rydberg
constant.
But one more question arises if you observe the hydrogen
spectrum closely.
How a single electron in the hydrogen atom manages to give an infinite number of spectral lines in the spectrum?
We may get confused by it. It is simple to understand. The
sample of hydrogen gas taken for study in the discharge tube contains one mole
of gaseous hydrogen atoms. While passing electric current, the different
hydrogen atoms absorb varying amounts of energy for their excitation. All of
them will give innumerable spectral emissions when they return to their
original stationary state. Moreover, the path of the electron journey is either
direct or may follow several intermediate steps to return to its initial
position. Under those circumstances, results in immeasurable spectral lines in
the hydrogen spectrum in various spectral series.
Fortunately, we have a simple formula to measure the number
of spectral lines in the spectrum.
 |
| The formula to calculate the number of spectral lines in the hydrogen spectrum |
Where,
n is principal quantum number.
The stationary orbit n=1 is close to the nucleus has the
lowest energy is said to be the permanent state of the system.
What is the maximum number of spectral lines emitted by a hydrogen atom when it is in the third excited state?
Here, we have principal quantum number= n=3
The number of spectral lines in the spectrum
 |
| The maximum number of spectral lines emitted in the third excited state |
Calculation of the wavelength of the hydrogen spectral line:
The energy possessed by an electron in the stationary orbit
is
 |
| The formula used to calculate the energy of the electron |
Consider that an excited electron jump
from energy level n2 with energy E2 to energy level n1 having energy E1. The
energy difference between these two stationary orbits proceeds with the
emission of the light radiation with frequency ϒ.
 |
| Calculation of the wavelength of the hydrogen spectral line |
Hydrogen spectrum diagram
In the energy diagram of the hydrogen spectrum, the
horizontal lines represent stationary levels of an electron in the atom. And
the vertical arrows in the downward direction represent the emission of energy
radiations during electron transitions. The electron absorbs energy equal to
the energy difference (∆E) between the two successive stationary orbits. Energy
less than ∆E is neither absorbed nor emitted by the electron during its
transmission.
 |
| The hydrogen spectrum diagram |
According to Neil Bohr's atomic theory, the permanent state is the state of an atom at which its principal quantum number value is one. In addition to that, it associates with the least energy among all stationary configurations of the atom.
Other static orbits have higher energy than the permanent
state. And they are known as the excited states. Notably, there are unlimited
excited energy levels in the atom. Consequently, the highest limit for the
excited orbits is immeasurable. For this reason, the energy diagram has only
one permanent state with an infinite number of higher energy orbits.
The hydrogen energy diagram indicates that the electron
transitions at higher static configurations occur at shorter wavelengths.
Why do the higher electron transitions emit light radiations of shorter wavelengths?
As we all know, electron transitions occur in the hydrogen
atom due to absorption immediately followed by the emission of energy in the
form of light radiation of a definite wavelength. The wavelength of these
emitted light radiation depends on the energy difference between the static
states involved in the electron transition.
According to the quantum theory of radiation, the energy
difference between the stationary orbits varies inversely with the wavelength
of the emitted light radiation.
 |
| The relationship between energy and wavelength of the light radiation |
So, the greater the energy difference, the shorter the wavelength of the emitted light radiation. To simplify, the electron transitions from higher energy levels emit photons at shorter wavelengths.
For example, the electron transition from third to second
transition state in the Balmer series gives a spectral line at 656 nm (longer
wavelength). But, the limiting line involving the electron transition from
infinite state to second energy level occurs at 365 nm (shorter wavelength).
Additional reference:
Why do short wavelengths have more energy than long wavelengths?
What do you mean by wavelength?
Which series of hydrogen spectrum will have the shortest wavelength?
The hydrogen spectral series that involves the long electron
transition will have the shortest wavelength. It will indicate the electron
journey from the top most energy level to the least stationary state in the
atom. Or the electron transference that is farther away from the nucleus to the
energy level closest to the nucleus (n=1).
Hence, the electron transition from an infinite stationary
state to n=1 in the Lyman series will have the shortest wavelength.
Importance of hydrogen spectral series
The first thing to remember is the hydrogen spectrum has
several spectral series. The spectral series consists of a sequence of spectral
lines arranged in the increasing order of their wavelengths. The Rydberg
formula measures the wavelengths of these spectral emissions in the hydrogen
atom.
According to Bohr's atomic theory, the electrons undergo
transitions between different stationary levels with the emission of photons of
definite wavelengths. The calculation of the wavelengths of these emitted light
radiations helps astronomers to detect the presence of hydrogen in the
celestial bodies and to measure their redshifts. In addition to that, spectral
studies help to determine the temperature and density of hydrogen gas in the
stars.
The splitting of spectral lines further clarifies the
strength of the magnetic field in the stars. If the spectral lines are fade in
strength, then it signifies the physical changes in the atom. The pattern and
width of spectral lines indicate the movement of orbiting stars.
Additional reference:
How do you determine the Rydberg constant using the hydrogen spectrum?
Final thoughts
We hope that this blog post is helpful
to you. And what else do you know about the hydrogen spectral series? Kindly share
your knowledge with us in the comments below. And also, you can visit our blog
https://jayamchemistrylearners.blogspot.com/ regularly for more engaging topics
of chemistry. We add new posts regularly to our blog. To not miss any updates,
kindly follow us. We will notify you immediately.
Most importantly, you can ask your
questions on the hydrogen spectral series topic in the post comment section and also
on our Instagram page. We are happy to hear from you and will answer you.
For more fascinating chemistry topic
visuals, please visit and follow our Instagram page @chemistrylearners and
Pinterest page @kameswariservices.